About Desmoid Tumours
Disease overview
An infiltrative and aggressive disease1
- Desmoid tumours are locally aggressive, potentially morbid tumours of the soft tissues, with a tendency to infiltrate surrounding structures and spread along planes and muscle1,2
- Sometimes referred to as aggressive fibromatosis or desmoid fibromatosis,3 these mesenchymal tumours can be serious, debilitating, and, in rare cases, even cause life-threatening organ damage2
- Desmoid tumours can infiltrate surrounding tissue, compressing muscles, nerves, and vessels4,5
Symptomatology and tumour sites
Desmoid tumours can cause severe and debilitating morbidities2
- Symptomatology related to desmoid tumours varies based on the location of tumour presentation5,6
- A prospective cohort study found that tumours in the chest wall, upper limb, and head and neck were associated with poor outcomes following surgery7
| TUMOUR SITE | ESTIMATED FREQUENCY5 | COMMON SYMPTOMS AND COMPLICATIONS |
|---|---|---|
| Intra-abdominal (including mesentery) | 20% | Compression may cause pain, cachexia, malaise, abdominal distension, or obstruction of the intestines or ureters5,8 |
| Abdominal Wall | 16% | Large tumours may cause tissue stretching, blood vessel compression, and bowel or bladder displacement9 |
| Lower Extremities | 16% | Limited mobility, pain, muscle stiffness, or deformity5,10 |
| Chest Wall | 15% | Dyspnea,5 dysphagia,5 pleural invasion,8 spinal11 or rib involvement,12 bone erosion,12 and pain12 |
| Upper Extremities | 14% | Restricted mobility, muscle and ligament involvement, limb weakness, deformity, or pain5,13 |
| Head and Neck | 8% | Pain, neurologic deficit, proximity to vital structures, including mortality risk from vascular or airway restriction14 |
| Other | 11% | Symptoms and complications dependent on the location5,6 |
The location of desmoid tumours can significantly affect quality of life.15 For example, a desmoid tumour with “tendril-like” growths1 that wrap around nerves may be associated with debilitating neuropathic pain.5,16
Disease course
Variable and unpredictable1
- Desmoid tumours are characterised by a variable and unpredictable disease course depending on tumour location and associated morbidity1,5
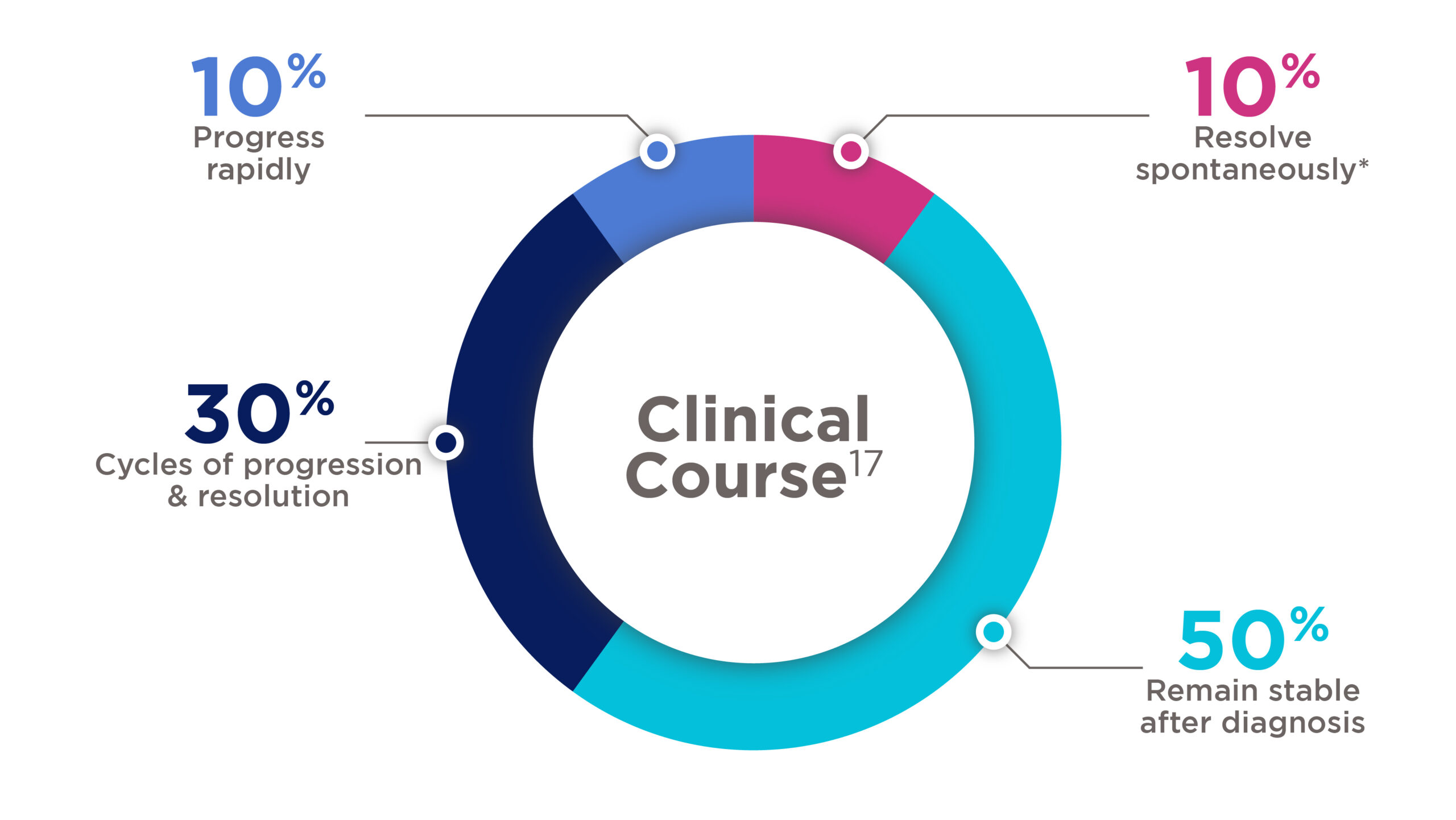
- Approximately, 50% of desmoids demonstrate an aggressive biology, continuing to grow or becoming symptomatic. The vast majority of progressions (89%) occurred within the first 2 years of observation4
Desmoid tumours clinical course
*10–28% of desmoid tumours will resolve spontaneously without treatment17
Incidence and risk factors
Epidemiology
Desmoid tumour has an incidence of approximately 5 cases per 1 million population per annum.18
The Orphanet Report Series provides an average annual incidence of 3 cases of desmoid tumours per million person-years in Europe.17
The epidemiological studies reveal that most desmoid tumour cases appear in the age range of 20–44 years.17
- Desmoid tumours are rare in young people and older adults19
- The incidence estimate has increased in more recent years, which may be related to an improvement in diagnostic techniques17
Female gender
Female-to-male ratio is about 2-3:1.20
- Pregnancy can promote desmoid tumours4
Antecedent trauma
About 25% of patients with desmoid tumours have a history of antecedent trauma.5
- Injury, or surgery may increase risk21
APC (adenomatous polyposis coli gene) mutations
Phenotypic characteristics correlate with the position of the APC mutation relative to certain codons.22
- Patients with familial adenomatous polyposis (FAP) have a 1000-fold higher risk of developing desmoid tumours than the general population20
ICD-10-CM codes for desmoid tumours
Aetiology and pathogenesis of desmoid tumours
Understanding molecular pathogenesis is of considerable interest in this disease given its highly variable clinical course19
The aetiology of desmoid tumours is unknown. However, the identification of clonal chromosomal changes in a significant fraction of cases supports the neoplastic nature of these tumours.19
- The pathogenesis of desmoid tumours is thought to be linked to trauma4
- surgery may sometimes promote growth of the tumour4
- growth factors released after surgery, during the initial phase of wound healing, could transmit signals that promote the activation of β-catenin4
- Pregnancy can promote DT not only secondary to hormonal influences, but also due to the systemic release of growth factors4
- Two pathways have been implicated in the canonical Wnt/β-catenin/APC pathway, in which CTNNB1 and APC mutations lead to β-catenin accumulation, and the Notch pathway, in which two cleavages at Notch receptors occur 17
- The potential crosstalk between the Notch and Wnt pathways, as well as activation of the Notch pathway resulting from dysregulation of the Wnt pathway, are thought to be involved in the pathogenesis of desmoid tumours17
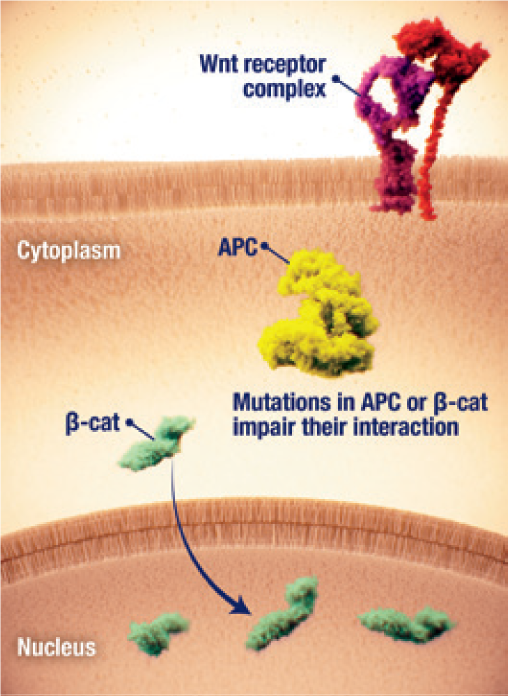
Nuclear accumulation of β-catenin
- Dysregulation of the Wnt/ β-catenin pathway has been implicated in the pathogenesis of desmoid tumours2,23
- β-catenin nuclear accumulation and downstream gene transcription is a key driver of desmoid tumour cell proliferation2,24
- Nuclear accumulation of β-catenin may be initiated by2,24
- Activating mutations of the β-catenin gene CTNNB1
- Inactivating mutations in the negative regulator APC, often in familial adenomatous polyposis (FAP)
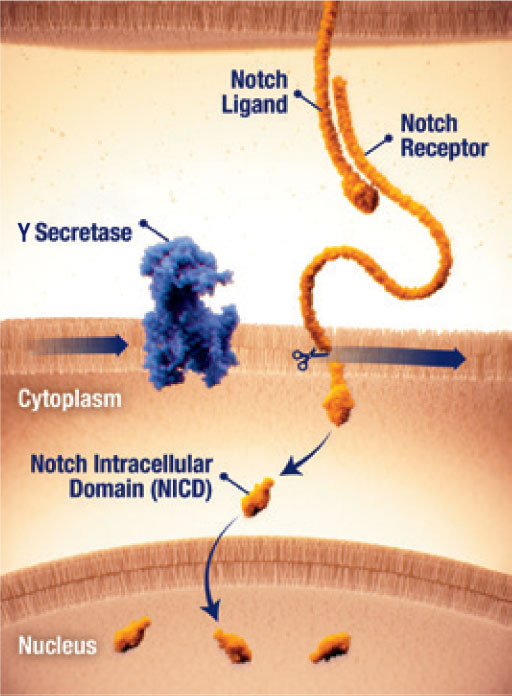
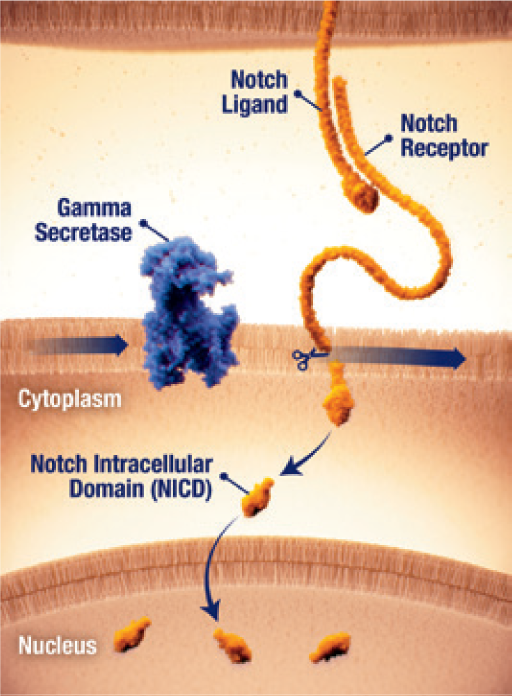
Notch activation by γ secretase
- The Notch pathway can be active in desmoid tumours25
- When dysregulated, Notch can activate pathways that contribute to tumour growth25
- Notch receptor signaling requires proteolytic activation by the enzyme γ secretase (gamma secretase)25
- γ secretase cleavage releases the Notch intracellular domain (NICD), which translocates to the nucleus and activates gene transcription25
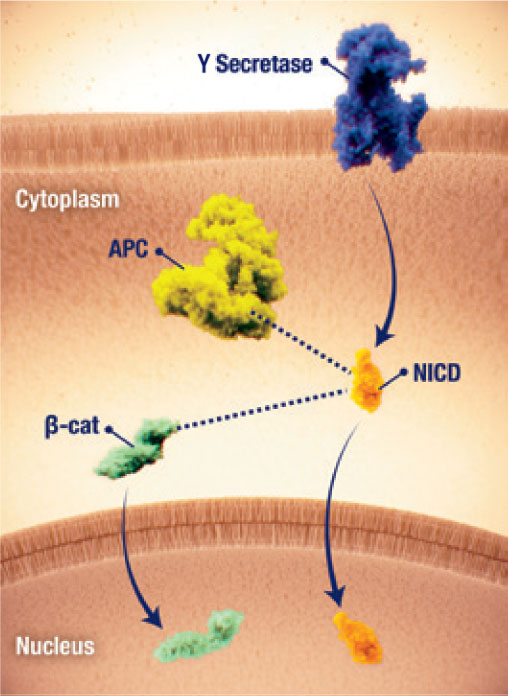
Pathway crosstalk
- Crosstalk between the Wnt/β-catenin and Notch pathways may further contribute to desmoid tumour pathogenesis17
- Dysregulation of the Wnt/ β-catenin pathway has been implicated in the pathogenesis of desmoid tumours2,24
- β-catenin nuclear accumulation and downstream gene transcription is a key driver of desmoid tumour cell proliferation2,25
- Nuclear accumulation of β-catenin may be initiated by2,25
- Activating mutations of the β-catenin gene CTNNB1
- Inactivating mutations in the negative regulator APC, often in familial adenomatous polyposis (FAP)
- The Notch pathway can be active in desmoid tumours26
- When dysregulated, Notch can activate pathways that contribute to tumour growth26
- Notch receptor signaling requires proteolytic activation by the enzyme γ secretase26
- γ secretase cleavage releases the Notch intracellular domain (NICD), which translocates to the nucleus and activates gene transcription26
- Crosstalk between the Wnt/β-catenin and Notch pathways may further contribute to desmoid tumour pathogenesis18
Risk of recurrence
- Recurrence rates can be influenced by tumour location:17
- Tumours on extremities are believed to be locally aggressive and have recurrence rates ranging from 24% to 77%
- Local recurrence rates of intra-abdominal tumours in patients with FAP are higher than those for extra-abdominal tumours and reported to be 57% to 86%
There is a high risk of local recurrence after surgery17
- Recurrence rates can be exacerbated by trauma, such as trauma from surgery, and range from approximately 25% to 60% at 5 years17
- The invasion of major vessels and nerves and quality of surgical margins are key factors for the high postoperative recurrence rate26
- Unfortunately, even when surgical margins are clear of tumour, recurrence rates are high27
Growth factors released after surgery during the initial wound-healing phase could promote β-catenin activation.4
- The fact that some tumours recurred after surgery but then remained stable without treatment suggests that growth factors released following surgery may have promoted recurrence in tumours that would otherwise have been indolent4
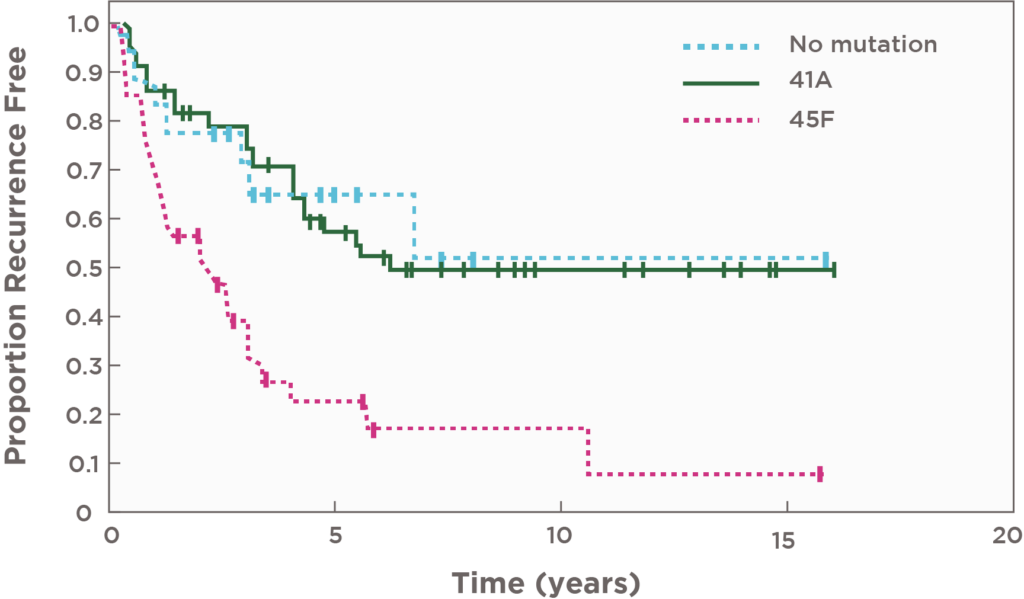
- The specific CTNNB1 mutation S45F seems to be associated with a worse recurrence-free survival after surgery28
- 5-year recurrence-free survival was significantly poorer in patients with S45F-mutated desmoid tumours (P<0.0001) vs patients with either T41A or nonmutated tumours29
| MUTATION | ESTIMATED RECURRENCE-FREE SURVIVAL (95% CI) |
|---|---|
| 45F | 23% (10%-40%) |
| 41A | 57% (43%-69%) |
| Non-mutated CTNNB1 | 65% (38%-83%) |
Patients with CTNNB1 mutations at codon 45 (45F) had a 3 times higher risk of recurrence post-surgery29
In a meta-analysis, tumour size was reported to be an important mediator for increased risk of recurrence in patients with the CTNNB1 S45F mutation.30
Principles of management
It is imperative to carefully select the management strategy for each patient with desmoid tumours to optimise tumour control and enhance quality of life18
Major efforts to standardize the management of this disease have been undertaken within the past decade.18
Currently, unmet needs in desmoid tumours include early and accurate diagnosis and approved treatments indicated for patients with desmoid tumours.17
In a study conducted at Memorial Sloan Kettering Cancer Center, 58% of patients with desmoid tumours converted from active surveillance to first-line treatment without radiographic tumour progression.31†
Treatment goals should not solely focus on clinical markers, such as progression-free survival, but also consider patient-relevant endpoints, such as a reduction in desmoid tumour-specific symptom burden (e.g., pain) and its impact on patients’ lives, improvement in functioning with daily activities, and overall quality of life.17
Early identification of progression
Identifying desmoid tumour progression can lead to timely initiation of appropriate treatment
- To help improve patient outcomes, healthcare professionals should assess for progression as early as possible with at least one of the following:
- Pain can be a prognostic indicator of progression34 and can be associated with worse outcomes35
- Risk of progression may be higher for larger tumours3,33
†Patients with primary or recurrent desmoid tumours (n=160) were identified retrospectively from an institutional database. Among the patients on initial observation for whom serial MRIs were available, 14 of 24 (58%) who underwent active treatment did not experience tumour growth as defined by RECIST criteria.31 Elaborated from text, reference 31.
Listen to a medical oncologist’s perspectives on desmoid tumours
Understanding desmoid tumours
Watch Dr Riedel, medical oncologist from Duke Health and Duke Cancer Institute, provide an overview of desmoid tumours, including pathophysiology, clinical presentations, and impact on patients.
APC, Adenomatosis Polyposis Coli gene; CT, Computed Tomography; CTNNB1, Catenin β1 gene; FAP, Familial Adenomatous Polyposis; MRI, Magnetic Resonance Imaging; NICD, Notch IntraCellular Domain; RECIST, Response Evaluation Criteria In Solid Tumors; S45F, mutation type; T41A, mutation type; Wnt, Wingless-related integration site.
References
- Kasper B et al. Desmoid Working Group. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma Patients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399-2408.
- Penel N et al. Adult desmoid tumors: biology, management and ongoing trials. Curr Opin Oncol. 2017;29(4):268-274.
- Gronchi A et al. Desmoid tumor working group. The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96-107.
- Bonvalot S et al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol. 2012;23(suppl 10):x158-x166.
- Constantinidou A et al. Clinical presentation of desmoid tumors. In: Litchman C, ed. Desmoid Tumors. Springer; 2012:chap 2. Accessed April 9, 2024. https://www.researchgate.net/publication/226455135.
- Joglekar SB et al. Current perspectives on desmoid tumors: the mayo clinic approach. Cancers (Basel). 2011;3(3):3143-3155.
- Penel N et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer. 2017;83:125-131.
- Shinagare AB et al. A to Z of desmoid tumors. AJR Am J Roentgenol. 2011;197(6):W1008-W1014.
- Koshariya M et al. Giant desmoid tumor of the anterior abdominal wall in a young female: a case report. Case Rep Surg. 2013;2013:780862.
- McDonald ES et al. Best cases from the AFIP: extraabdominal desmoid-type fibromatosis. Radiographics. 2008;28(3):901-906.
- Abrão FC et al. Desmoid tumors of the chest wall: surgical challenges and possible risk factors. Clinics (Sao Paulo). 2011;66(4):705-708.
- Xie Y et al. Recurrent desmoid tumor of the mediastinum: a case report. Oncol Lett. 2014;8(5):2276-2278.
- Scaramussa FS et al. Desmoid tumor in hand: a case report. SM J Orthop. 2016;2(3):1036.
- Baranov E et al. Soft tissue special issue: fibroblastic and myofibroblastic neoplasms of the head and neck. Head Neck Pathol. 2020;14(1):43-58.
- Ingley KM et al. High prevalence of persistent emotional distress in desmoid tumor. Psychooncology. 2020;29(2):311-320.
- Gounder MM et al. Prospective development of a patient-reported outcomes instrument for desmoid tumors or aggressive fibromatosis. Cancer. 2020;126(3):531-539.
- Bektas M et al. Desmoid tumors: a comprehensive review. Adv Ther. 2023;40(9):3697-3722.
- Kasper B et al. Desmoid Tumor Working Group. Current management of desmoid tumors: a review. JAMA Oncol. 2024;10(8):1121-1128.
- Ravi V et al. Desmoid tumors: epidemiology, molecular pathogenesis, clinical presentation, diagnosis, and local therapy Jun 2024 UptoDate.
- Skubitz KM. Biology and treatment of aggressive fibromatosis or desmoid tumor. Mayo Clin Proc. 2017;92(6):947-964.
- Lopez R et al. Problems in diagnosis and management of desmoid tumors. Am J Surg. 1990;159(5):450-453.
- Kasper B et al. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16(5):682-93.
- Crago AM et al. Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fi bromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer. 2015;54(10):606-615.
- Gronchi A et al. Desmoid tumor working group. The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients [supplementary appendix]. Eur J Cancer. 2020;127:96-107.
- Shang H et al. Targeting the Notch pathway: a potential therapeutic approach for desmoid tumors. Cancer. 2015;121(22):4088-4096.
- Wang YF et al. Postoperative recurrence of desmoid tumors: clinical and pathological perspectives. World J Surg Oncol. 2015;13:26.
- Easter DW et al. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg. 1989;210(6):765-769.
- Napolitano A et al. Recent advances in desmoid tumor therapy. Cancers (Basel). 2020;12(8):2135.
- Lazar AJ et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173(5):1518-27.
- Timbergen MJM et al. The prognostic role of β-Catenin mutations in desmoid-type fibromatosis undergoing resection only: a meta-analysis of individual patient data. Ann Surg. 2021;273(6):1094-1101.
- Cassidy MR et al. Association of MRI T2 signal intensity with desmoid tumor progression during active observation: a retrospective cohort study. Ann Surg. 2020;271(4):748-755.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcoma V.1.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed April 29, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Kasper B et al. Desmoid Tumor Working Group. Current management of desmoid tumors: a review. [supplementary appendix] JAMA Oncol. 2024;10(8):1121-1128.
- Cuomo P et al. Extra-abdominal desmoid tumor fibromatosis: a multicenter EMSOS study. BMC Cancer. 2021;21(1):437.
- Penel N et al. Pain in desmoid-type fibromatosis: prevalence, determinants and prognosis value. Int J Cancer. 2023;153(2):407-416.